
Empirical Formula Worksheet Pdf worksheet
Liveworksheets transforms your traditional printable worksheets into self-correcting interactive exercises that the students can do online and send to the teacher.. Grade 10,Empirical and molecular formula Grade 10,Empirical and molecular formula. Loading ad. ADAWIYA FATAYER. Member for 2 years 10 months Age: 16-18. Level: 10. Language:.

Working With Formulas Worksheet
Empirical/Molecular Formula Practice Worksheet Directions: Find the empirical AND molecular formulas given the percent compositions or masses. SHOW YOUR WORK to receive full credit 1) 26,4% Carbon 3.3 % Hydrogen 70.3 % Oxygen Molar Mass: 91.0 g/mol Empirical Formula: Molecular Formula: 2) 81.8 grams Carbon 18.2 grams Hydrogen Molar Mass: 132,0.

Empirical and Molecular Formula Practice Solutions Docsity
Empirical Formula Practice Test Questions The empirical formula is the simplest whole-number ratio of the elements. This practice exam tests finding empirical formulas of chemical compounds. The empirical formula is the simplest whole-number ratio of the elements. This practice exam tests finding empirical formulas of chemical compounds. Menu Home

Empirical Formula Practice Worksheet
Objectives: • be able to calculate empirical and molecular formulas Empirical Formula 1) What is the empirical formula of a compound that contains 0.783g of Carbon, 0.196g of Hydrogen and 0.521g of Oxygen? 2) What is empirical formula of a compound which consists of 89.14% Au and 10.80% of O?
Empirical Formula Worksheet 1 Answers
Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)

8 Best Images of Percent Composition Worksheet Answer Key Percent
Empirical/Molecular Formula Practice Worksheet Directions: Find the empirical AND molecular formulas given the percent compositions or masses. SHOW YOUR WORK to receive full credit. 1) 26.4 % Carbon 3.3 % Hydrogen 70.3 % Oxygen Empirical Formula:_________________ 2) 81.8 grams Carbon 18.2 grams Hydrogen Empirical Formula:_________________

formula writing worksheet answers
What is the empirical formula of the product? Chapter 7 - The Simplest, or Empirical, Formula Section A Determine the empirical formula for each compound whose percentage composition is shown below. 43% C and 57% O 40.3% K, 26.7% Cr, and 33.0% O 32.0% C, 42.6% O, 18.7% N, and the remainder H 31.9% K, 28.9% Cl, and the remainder O

Determining Empirical Formulas Worksheet Printable Word Searches
Step 1: If you are given percent composition pretend the percents are grams and go to the next step. (Really- just change the % to a g for grams!!!) Step 2: Convert the mass of each element to moles of each element using the atomic masses from the periodic table.

Empirical Formula Practice Worksheet
Practice: Empirical and Molecular Formulas. Explore and practice Nagwa's free online educational courses and lessons for math and physics across different grades available in English for Egypt. Watch videos and use Nagwa's tools and apps to help students achieve their full potential.

Empirical and Molecular Formula Practice by Teach Simple
Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)
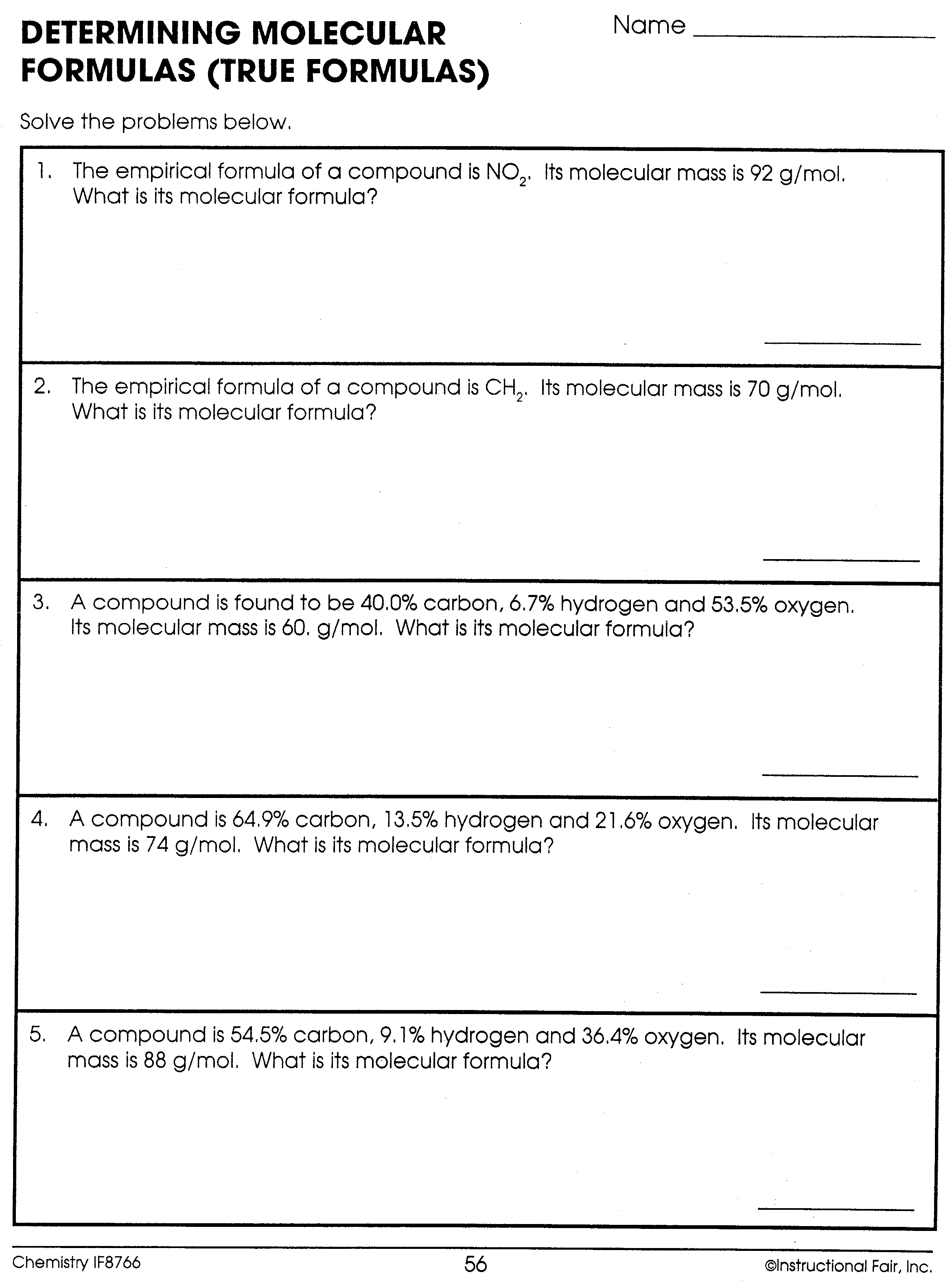
Empirical And Molecular Formula Problems Pdf UPDATED
Empirical and Molecular Formulas. Exercise 7.2.3.1 7.2.3. 1. For each formula below, give the empirical formula. Sometimes the formula given is the same as the empirical, sometimes it is different. a) C 3 H 6 O 3. b) N 2 O 4. c) Mg 3 N 2. d) C 7 H 14 O 2. e) P 2 O 5.

Percent Composition By Mass Worksheet
Formula Practice worksheets We came up with two significant sorts of chemical formulas: molecular formula and empirical formula, by calculating the masses of all the constituent atoms that combine to create a molecule.

Empirical/molecular Formula Practice Worksheet Answer Key Printable
Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C 6H 6 2) C8H18 3) WO2 4) C2H6O2 5) X 39Y 13 6) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. What is the molecular formula of this compound?

Empirical and Molecular Formula Worksheet Write the empirical formula
This 10-question practice test deals with finding the molecular formula of chemical compounds. A periodic table will be required to complete this test. Answers appear after the final question. Question 1 An unknown compound is found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen with a molecular mass of 60.0 g/mol.

Empirical and Molecular Formula Practice Problems
1) 26.4% Carbon 3.3 % Hydrogen 70.3 % Oxygen Molar Mass: 91.0 g/mol Empirical Formula: Molecular Formula: 2) 81.8 grams Carbon 18.2 grams Hydrogen Molar Mass: Here's the best way to solve it. Empirical/Molecular Formula Practice Worksheet Directions: - questions to Find the empirical AND molecular formulas given the percent compositions or.

Empirical And Molecular Formulas Worksheet
The molecular mass of the compound is 58.12 g/mol. Determine the molecular formula. 8 min max PAGE 349, QUESTION 145 145) Determine the molecular formula for ibuprofen, a common headache remedy. Analysis of ibuprofen yields a molar mass of 206 g/mol and a percent composition of 75.7% C, 8.80% H, and 15.5% O. Determine the molecular formula. 10.